
- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
The Study on Reaction-Sintered SiC Ceramics and Their Properties
2024-09-24
Why Is Silicon Carbide Important?
Silicon carbide (SiC) is a compound formed by covalent bonds between silicon and carbon atoms, known for its excellent wear resistance, thermal shock resistance, corrosion resistance, and high thermal conductivity. It is widely used in aerospace, mechanical manufacturing, petrochemicals, metal smelting, and the electronics industry, particularly for making wear-resistant parts and high-temperature structural components. Reaction-sintered silicon carbide ceramics are among the first structural ceramics to achieve industrial-scale production. Traditional reaction-sintered silicon carbide ceramics are made from silicon carbide powder and a small amount of carbon powder through high-temperature silicon infiltration reaction sintering, which requires long sintering times, high temperatures, high energy consumption, and high costs. With the growing application of reaction-sintered silicon carbide technology, traditional methods are insufficient to meet the industrial demand for complex-shaped silicon carbide ceramics.
What Are Recent Advances in Reaction-Sintered Silicon Carbide?
Recent advances have led to the production of high-density, high-bending-strength silicon carbide ceramics using nano-sized silicon carbide powder, significantly improving the material’s mechanical properties. However, the high cost of nano-sized silicon carbide powder, priced at over tens of thousands of dollars per ton, hinders large-scale application. In this work, we used widely available wood charcoal as the carbon source and micron-sized silicon carbide as the aggregate, employing slip casting technology to prepare reaction-sintered silicon carbide ceramic green bodies. This approach eliminates the need for pre-synthesizing silicon carbide powder, reduces production costs, and enables the fabrication of large, complex-shaped thin-walled products, providing a reference for improving the performance and application of reaction-sintered silicon carbide ceramics.
What Were the Raw Materials Used?
The raw materials used in the experiment include:
Silicon carbide with a median particle size (d50) of 3.6 μm and purity (w(SiC)) ≥ 98%
Carbon black with a median particle size (d50) of 0.5 μm and purity (w©) ≥ 99%
Graphite with a median particle size (d50) of 10 μm and purity (w©) ≥ 99%
Dispersants: Polyvinylpyrrolidone (PVP) K30 (K value 27-33) and K90 (K value 88-96)
Water reducer: Polycarboxylate CE-64
Release agent: AO
Deionized water
How Was the Experiment Conducted?
The experiment was conducted as follows:

Mixing the raw materials according to Table 1 using an electric mixer for 4 hours to obtain a uniformly mixed slurry.
Keeping the slurry viscosity ≤ 1000 mPa·s, the mixed slurry was poured into prepared gypsum molds for slip casting, allowed to dehydrate through the gypsum molds for 2-3 minutes to form green bodies.
The green bodies were placed in a cool location for 48 hours, then removed from the molds, and dried in a vacuum drying oven at 80°C for 4-6 hours.
Degumming of the green bodies was performed in a muffle furnace at 800°C for 2 hours to obtain the preforms.
The preforms were embedded in a mixed powder of carbon black, silicon powder, and boron nitride in a mass ratio of 1:100:2000, and sintered in a furnace at 1720°C for 2 hours to obtain fully fine-powdered silicon carbide ceramics.
What Methods Were Used for Performance Testing?
Performance testing included:
Measuring the viscosity of the slurry at various mixing times (1-5 hours) using a rotary viscometer at room temperature.
Measuring the volume density of the preforms following the national standard GB/T 25995-2010.
Measuring the bending strength of the sintered samples at 1720°C according to GB/T 6569-2006, with sample dimensions of 3 mm × 4 mm × 36 mm, span of 30 mm, and loading speed of 0.5 mm·min^-1.
Analyzing the phase composition and microstructure of the sintered samples at 1720°C using XRD and SEM.
How Does Mixing Time Affect the Slurry Viscosity, Preform Volume Density, and Apparent Porosity?
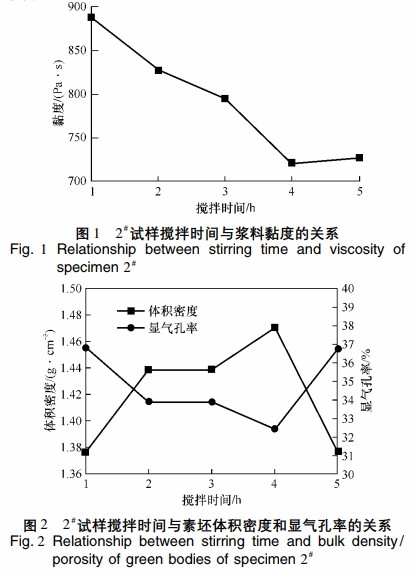
Figures 1 and 2 respectively show the relationship between mixing time and slurry viscosity for sample 2#, and the relationship between mixing time and preform volume density and apparent porosity.
Figure 1 indicates that as mixing time increases, viscosity decreases, reaching a minimum of 721 mPa·s at 4 hours and then stabilizes.
Figure 2 shows that sample 2# has a maximum volume density of 1.47 g·cm^-3 and a minimum apparent porosity of 32.4%. Lower viscosity results in better dispersion, leading to more uniform slurry and improved silicon carbide ceramic performance. Insufficient mixing time leads to uneven mixing of silicon carbide fine powder, while excessive mixing time evaporates more water, destabilizing the system. The optimal mixing time for preparing fully fine-powdered silicon carbide ceramics is 4 hours.
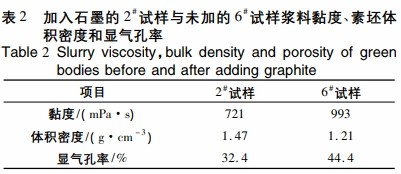
Table 2 lists the slurry viscosity, preform volume density, and apparent porosity of sample 2# with added graphite and sample 6# without added graphite. The addition of graphite lowers slurry viscosity, increases preform volume density, and reduces apparent porosity due to graphite’s lubricating effect, resulting in better dispersion and increased density of fully fine-powdered silicon carbide ceramics. Without graphite, the slurry has higher viscosity, poorer dispersion, and stability, making graphite addition necessary.
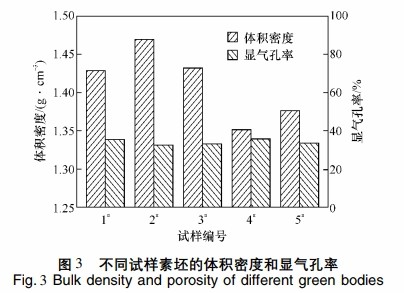
Figure 3 displays the preform volume density and apparent porosity of samples with different carbon black contents. Sample 2# has the highest volume density of 1.47 g·cm^-3 and the lowest apparent porosity of 32.4%. However, too low porosity impedes silicon infiltration.
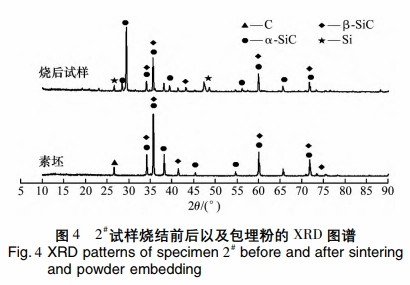
Figure 4 shows the XRD spectra of sample 2# preforms and sintered samples at 1720°C. The preforms contain graphite and β-SiC, while the sintered samples contain Si, β-SiC, and α-SiC, indicating some β-SiC transformed into α-SiC at high temperatures. The sintered samples also show increased Si and decreased C content due to high-temperature silicon infiltration, where Si reacts with C to form SiC, filling the pores.

Figure 5 shows the fracture morphology of different sample preforms. The images reveal fine silicon carbide, graphite, and pores. Samples 1#, 4#, and 5# have larger flake phases and more unevenly distributed pores due to uneven mixing, resulting in low preform density and high porosity. Sample 2# with 5.94% (w) carbon black shows optimal microstructure.

Figure 6 shows the fracture morphology of sample 2# after sintering at 1720°C, displaying tightly and uniformly distributed silicon carbide particles with minimal porosity. The growth of silicon carbide particles is due to high-temperature effects. Smaller newly-formed SiC particles are also seen between the original SiC skeleton particles from reaction sintering, with some residual Si filling the original pores, reducing stress concentration but potentially affecting high-temperature performance due to its low melting point. The sintered product has a volume density of 3.02 g·cm^-3 and a bending strength of 580 MPa, over twice the strength of ordinary reaction-sintered silicon carbide.
Conclusions
The optimal mixing time for the slurry used to prepare fully fine-powdered silicon carbide ceramics is 4 hours. Adding graphite reduces slurry viscosity, increases preform volume density, and decreases apparent porosity, enhancing the density of fully fine-powdered silicon carbide ceramics.
The optimal carbon black content for preparing fully fine-powdered silicon carbide ceramics is 5.94% (w).
The sintered silicon carbide particles are tightly and uniformly distributed with minimal porosity, showing a growth trend. The sintered product density is 3.02 g·cm^-3, and the bending strength is 580 MPa, significantly improving the mechanical strength and density of fully fine-powdered silicon carbide ceramics.**
We at Semicorex specialize in SiC Ceramics and other Ceramic Materials applied in semiconductor manufacturing, if you have any inquiries or need additional details, please don't hesitate to get in touch with us.
Contact phone: +86-13567891907
Email: sales@semicorex.com




